

Firstly, the stability and reactivity of compounds were determined by using the Guassian09 programme in which the density functional theory (DFT) calculations were performed by using the B3LYP/SVP level. This is the first comprehensive study which focused on the identification of phenylcarbamoylazinane-1, 2,4-triazole amides (7a–o) as the inhibitors of aldo-keto reductases (AKR1B1, AKR1B10) via detailed computational analysis. The results suggested that these derivatives can be utilized in future for the synthesis of more potent inhibitors of alkaline phosphatase that can be used in future for treating different types of cancer especially breast cancer.īoth members of the aldo-keto reductases (AKRs) family, AKR1B1 and AKR1B10, are over-expressed in various type of cancer, making them potential targets for inflammation-mediated cancers such as colon, lung, breast, and prostate cancers. The docking energies of 4c and 4g are highest among all the compounds i.e., −32.18 and −30.09 kJ/mol, respectively. The outcome of this study suggests that the synthesized compounds are potent alkaline phosphatase inhibitors whereas 4c and 4g are best compounds with lowest IC50 value i.e., 0.057 and 0.019 µM, respectively. The density functional theory (DFT) studies and molecular docking studies of synthesized derivatives were carried out to understand the reactivity profile and binding behavior of all the compounds. ¹H and ¹☼ NMR were utilized for identification of the chemical structures of all the synthesized compounds. Using all the standard protocols, the synthesized compounds were assessed for alkaline phosphatase (AP) assay. These are also commonly referred to as HOMO−1 and LUMO+1 respectively.This study was carried out to identify the synthesis of a new group of thiazole-linked thioureas with aromatic and aliphatic side chains (4a–k) using a one-pot three-component strategy which are well known for their material and medicinal properties. They are named NHOMO for next-to-highest occupied molecular orbital and SLUMO for second lowest unoccupied molecular orbital. If existent, the molecular orbitals at one energy level below the HOMO and one energy level above the LUMO are also found to play a role in frontier molecular orbital theory. This abbreviation may also be extended to semi occupied molecular orbital. In organometallic chemistry, the size of the LUMO lobe can help predict where addition to pi ligands will occur.Ī SOMO is a singly occupied molecular orbital such as half-filled HOMO of a radical. The same analogy can be made between the LUMO level and the conduction band minimum.
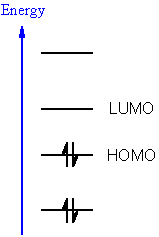
The HOMO level is to organic semiconductors roughly what the maximum valence band is to inorganic semiconductors and quantum dots. As a rule of thumb, the larger a compound's HOMO-LUMO gap, the more stable it is. The size of this gap can be used to predict the strength and stability of transition metal complexes, as well as the colors they produce in solution. The energy difference between the HOMO and LUMO is termed the HOMO–LUMO gap. 5 Subadjacent orbitals: NHOMO and SLUMO.


 0 kommentar(er)
0 kommentar(er)
